An FDA pivotal trial of our hospital monitoring device is underway at multiple sites, following promising first-in-human pilot studies and foundational research.
Clinical Evidence for Sense Technology
First-in-Human ICH monitor trial

9 patients enrolled

600 hours of monitoring time

3600 SENSE scans performed


7 no expansion
100% correspondence of SENSE with CT results at 12 hours. No false positives/no false negatives.
First-in-Human Stroke differentiation trial

5 LVO*, 5 non LVO*, 5 ICH**

Study conducted at Good Samaritan Hospital and UC

5 LVO*(completed)

5 non-LVO* completed

5 ICH** completed
**Intracerebral hemorrhage (ICH)
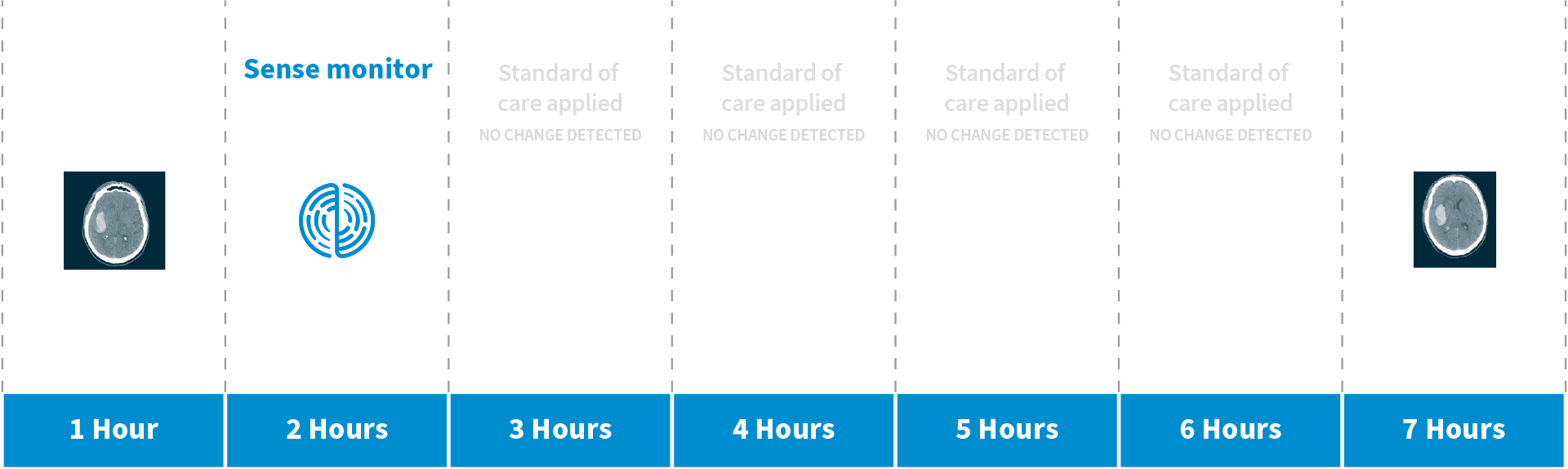
Case Study: Hospital Monitoring
Patient data from our 2020 First-in-Human ICH monitor trial demonstrates Sense’s NeuSTAT™ non-invasive, continuous monitoring device detected a 3 ml expanded bleed five hours before it was detected through the current standard of care.
Contact Us
We are driven by our mission and are committed to impacting people’s lives and enhancing neurological health globally. You can be part of our journey too.
Copyright Sense Neuro 2021 All Rights Reserved. Designed by Node
